electron configuration of radon|radon bohr model : Clark The Electron configuration of Radon is Xe 4f14 5d10 6s2 6p6. Radon is a chemical element which is represented by the symbol Rn and whose atomic number corresponds to 86. .
REGION 7. CENTRAL VISAYAS. Central Visayas, officially designated as Region VII, is an administrative region in the Philippines occupying the central section of the Visayas.It covers 4 provinces, namely, Bohol, .
PH0 · zr4+ electron configuration
PH1 · rn valence electrons
PH2 · radon valence electrons
PH3 · radon number of electrons
PH4 · radon bohr model
PH5 · full electron configuration of platinum
PH6 · electron configuration explained
PH7 · Iba pa
19 Years Old. 19 years old, famous university ⚫︎, current Ekisupo girl, former national athlete, 168cm, gravure body type, beautiful big E-cup tits!
electron configuration of radon*******The arrangement of electrons in radon in specific rules in different orbits and orbitals is called the electron configuration of radon. The electron configuration of radon is [ Xe ] 4f 14 5d 10 6s 2 6p 6 , if the electron arrangement is through orbitals. Wayne Breslyn. 784K subscribers. 142. 16K views 3 years ago. A step-by-step description of how to write the electron configuration for Radon (Rn). In order to .Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. . Radon condensed electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6. It is the same as the ground state .
Radon is a radioactive noble gas with atomic number 86 and symbol Rn. Its electron configuration is [Xe] 4f 14 5d 10 6s 2 6p 6, with 8 valence electrons and 0 oxidation states.The Electron configuration of Radon is Xe 4f14 5d10 6s2 6p6. Radon is a chemical element which is represented by the symbol Rn and whose atomic number corresponds to 86. .Its first ionization energy—the minimum energy required to extract one electron from it—is 1037 kJ/mol. In accordance with periodic trends, radon has a lower electronegativity than the element one period before .
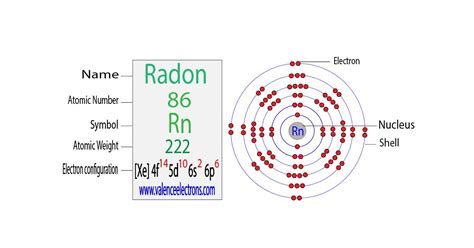
Radon is a chemical element with atomic number 86 which means there are 86 protons and 86 electrons in the atomic structure. The chemical symbol for Radon is .Atomic Structure of Radon. Atomic Radius: 1.34 Å. Atomic Volume: 50.5cm 3 / mol. Covalent Radius: Cross Section (Thermal Neutron Capture) σ a / barns: 0.72. Crystal .Electron Configuration. [Xe] 4f 14 5d 10 6s 2 6p 6. Rn. Upon condensation, radon glows because of the intense radiation it produces. Physical Properties.
The electronic configuration of Radon is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6. What is the abbreviated electronic configuration of Radon? The abbreviated electronic configuration . Radon -. Rn: properties of free atoms. Radon atoms have 86 electrons and the shell structure is 2.8.18.32.18.8. The ground state electron configuration of ground state gaseous neutral radon is [ Xe .Answer: The electron configurations of the elements are presented in Figure 2.2.3, which lists the orbitals in the order in which they are filled. In several cases, the ground state electron configurations are different from those predicted by Figure 2.2.1. Some of these anomalies occur as the 3 d orbitals are filled.Electron configuration 4f 14 5d 10 6s 2 6p 6: Electrons per shell: 2, 8, 18, 32, 18, 8: Physical properties; . Radon is a chemical element; it has symbol Rn and atomic number 86. It is a radioactive noble gas and is colorless and odorless. Of the three naturally occurring radon isotopes, . Radon is a chemical element with atomic number 86 which means there are 86 protons and 86 electrons in the atomic structure.The chemical symbol for Radon is Rn. Electron Configuration and Oxidation States of Radon. Electron configuration of Radon is [Hg] 6p6. Possible oxidation states are 0. Electron Configuration

ラドンの電子配置. ラドンの電子配置はXe4f14d5s10p6です。. ラドンは、記号Rnで表され、原子番号が2に対応する化学元素です。. ラドンには、6Rnから6Rまでの合計86の同位体があります。. それは希ガスガスを構成するものの中で最も重く、その同位体のそれぞれ .
What is The Electron Configuration of Radon? Xe 4f14 5d10 6s2 6p6 is the electron configuration of the radon. How Many Valence Electrons Does Radon Have. Radon has eight valence electrons in its outer shell. Radon Number of Valence Electrons.electron configuration of radon radon bohr modelLa configuración electrónica de Radon es Xe 4f14 5d10 6s2 6p6. El radón es un elemento químico que se representa con el símbolo Rn y cuyo número atómico corresponde a 86. Hay un total de 37 isótopos del mismo, que se conocen desde el 195Rn hasta el 231R. Es el más pesado de los que componen los gases nobles, y cada uno de sus isótopos .
Is there a promotional code that Neospin Casino requires? No, you are not required to input any bonus codes at the casino. Are there any withdrawal limits at Neospin Casino? The daily withdrawal limit at Neospin Casino is 5,000 EUR. Is it possible to play Neospin Casino on a mobile device? Yes, Neospin Casino is completely mobile-friendly!The Crossword Solver found 30 answers to "timber wolf, 4", 4 letters crossword clue. The Crossword Solver finds answers to classic crosswords and cryptic crossword puzzles. Enter the length or pattern for better results. Click the answer to find similar crossword clues.
electron configuration of radon|radon bohr model